We combine an experienced Management Team with locally based staff, providing the optimum combination of customer service with local knowledge.
We have an exceptional team of Clinical Trial Managers, CRAs, SSU Specialists and Regulatory experts, all of whom are committed in running successful studies.
WHAT MAKES
THE DIFFERENCE
- THE TEAM
- OUR EXPERIENCE
- OUR PERFORMANCE
- START-UP TIMELINES
- HIGH RECRUITMENT RATES
- SOLVING PROBLEMS
- SINGLE POINT OF CONTACT
- OUR QUALITY
EXCELLENCE, QUALITY AND EXPERTISE

OUR TEAM
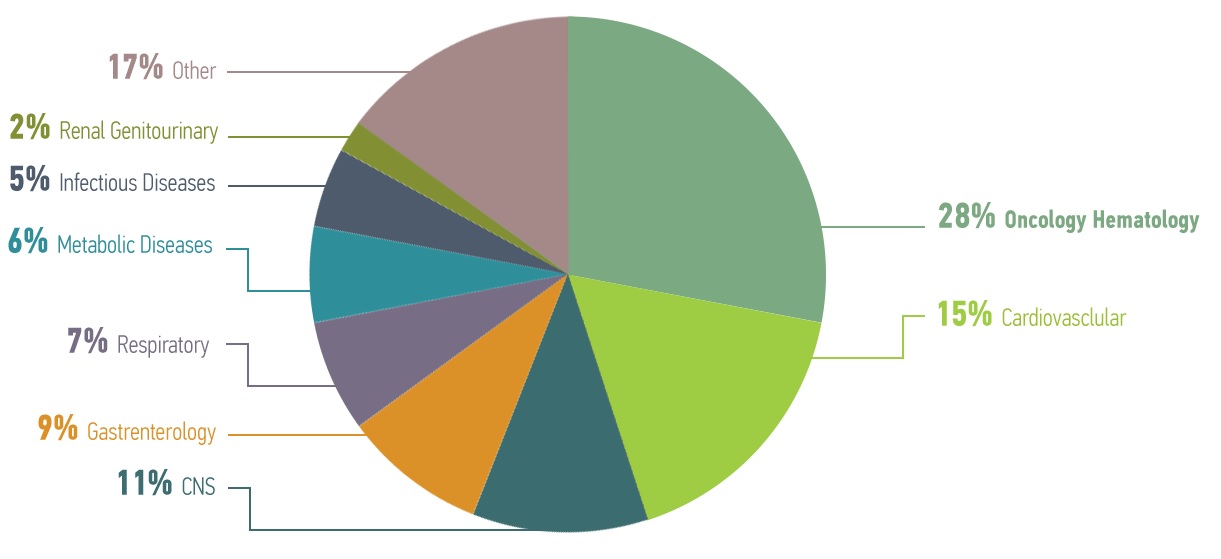
OUR EXPERIENCE PER THERAPEUTIC AREA
Being a company of dynamic professionals, offering contract services, through reliable partnerships, excellence, quality and expertise in highly specialized fields, we collaborate with all stakeholders across the biopharmaceutical research and development community, in all therapeutic areas
OUR EXPERIENCE PER PHASE
Phase I 2% / Phase II 25% / Phase III 45% / Phase IV 28%
OUR CLIENTS
Biotech 30% / Pharmaceutical 20% / Medical Device 10% / CROs 30% / Research Institutes- Academic 10%
OUR PERFORMANCE
80
In 80% of the global studies that we participate, we manage to be the first to initiate a site
70
In 70% of the global studies that we participate, we managed to have one site as top recruiter
80
80% of the initiated sites are recruiting
100
100% we specialize in rare diseases
- We speed up timelines by 15%
- We exceed our recruitment targets by 30%

WE COMMIT ON
- HIGH SPONSOR’S EXPECTATIONS/KPIs
- FAST START-UP TIMELINES
- DEDICATED STUDY TEAMS
- ADEQUATE RESOURCES
- SHARING COUNTRY INTELLIGENCE
- “PLUG & PLAY” CRAs
CHECK IN FOR YOUR
GLOBAL STUDIES
NEXT CRO is a Contract Research Organisation (CRO), running Clinical Trials in Greece, Turkey and Cyprus and, with our alliances, globally
YOU CAN CHECK-IN WITH NEXT CRO FOR YOUR GLOBAL STUDIES